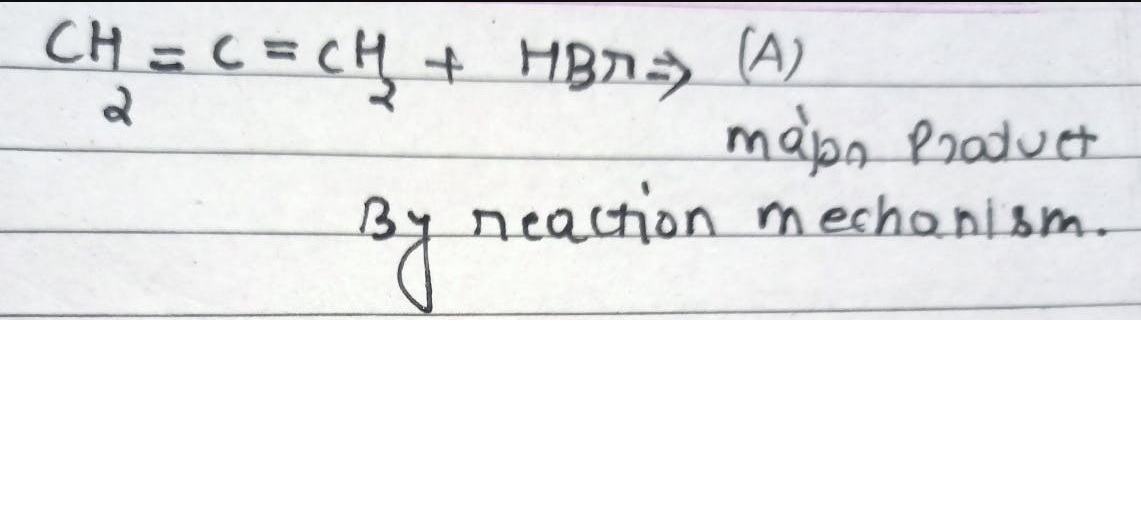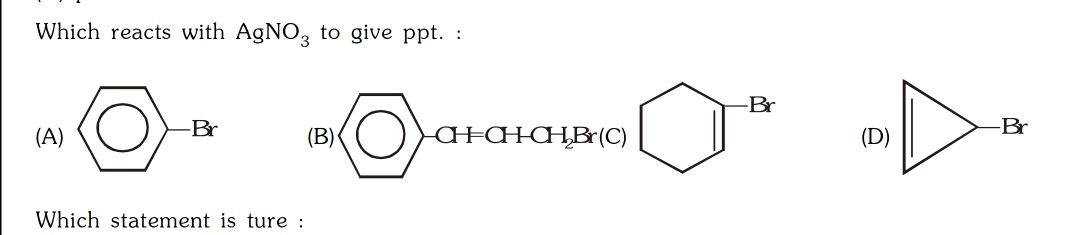CBSE Class 12-science Answered
why does swartz reaction does not need any reagent ?
Asked by kpsingh769 | 02 Jun, 2017, 11:05: AM
In Swarts’ reaction, heavy metal fluorides are used as reagents.
It is a reaction in which alkyl fluorides are formed when alkyl bromide or chloride reacts with metal fluorides.
In the reaction, higher alkyl halide(R-Cl) react with lower metal halide (AgF) to form lower alkyl halide(R-F).
If we don’t use heavy metal fluoride F ion cannot displace Cl/Br.
The reaction will not occur or it may form some other compound.
Answered by Vaibhav Chavan | 02 Jun, 2017, 06:26: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM