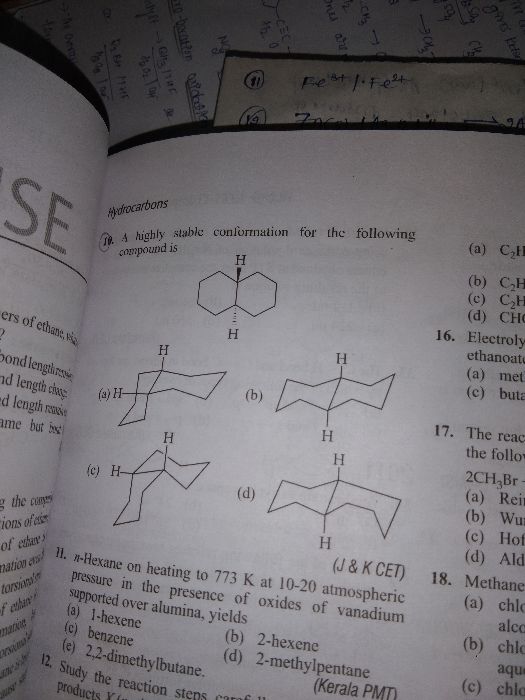
CBSE Class 11-science Answered
WHY DO WE GET ISOPROPYL BENZENE ON TREATING BENZENE WITH 1-CHLOROPROPANE INSTEAD OF N-PROPYL BENZENE?
Asked by kuldeep shrivastava | 21 Feb, 2011, 12:27: PM
Dear Student
This is an example of electrophilic substitution reaction. The 1-chloropropane produces n-propyl cation which stabilises through reonance and produces 2-propyl cation.
Since 1º-carbocations are prone to rearrangement, it is usually not possible to introduce 1º-alkyl substituents larger than ethyl by Friedel-Crafts alkylation. So, reaction of excess benzene with 1-chloropropane and aluminum chloride gives a good yield of isopropylbenzene (cumene).
C6H6 (large excess) + CH3CH2CH2-Cl + AlCl3 ——> C6H5-CH(CH3)2 + HCl
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 21 Feb, 2011, 07:54: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM