ICSE Class 10 Answered
Why direct iodination of methane is not carried.Then how is it carried
Asked by lovemaan5500 | 17 Dec, 2017, 06:33: AM
Iodine reacts with methane reversibly. When iodine reacts with methane, it gives methyl iodide and hydrogen iodide which is a strong reducing agent and it can convert to iodomethane back to methane.
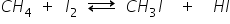
To overcome this difficulty iodination is carried out in presence of strong oxidizing agents like HIO3 which oxidize HI formed during the reaction.
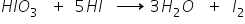
Answered by Ramandeep | 17 Dec, 2017, 11:33: PM
Concept Videos
ICSE 10 - Chemistry
Asked by shalokrajkumar | 17 May, 2020, 09:17: AM
ICSE 10 - Chemistry
Asked by Sirfria | 18 Feb, 2020, 10:12: PM
ICSE 10 - Chemistry
Asked by priyankasharma | 19 Mar, 2018, 06:24: PM
ICSE 10 - Chemistry
Asked by gpnkumar0 | 14 Mar, 2018, 09:08: PM


