CBSE Class 12-science Answered
Why decrease in the activation energy leads to an increase in the rate of reaction?
Asked by Nishtha Sardana | 18 Jun, 2014, 04:50: PM
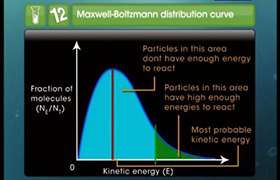
In the formation of product from reactant, it has to go through an intermediate stage. This stage is called transition state. The energy required for the formation of transition state from reactant is called activation energy.
If the activation energy for the reaction is lower, that causes reactant to follow a shorter path as shown in second diagram (Curve marked with pink colour). Thus, as a result the reactant has to cover a shorter distance in forming the product, it would require less time and in turn the rate of reaction will increase.
Answered by Prachi Sawant | 20 Jun, 2014, 12:24: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by imtiyazmulla68 | 22 Mar, 2018, 08:25: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:56: AM