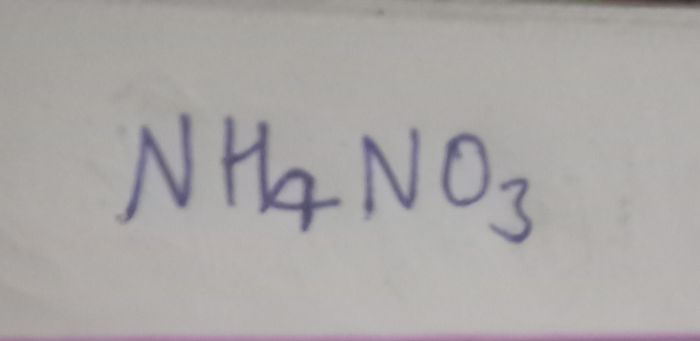CBSE Class 12-science Answered
Why can't bigger elements such as phosphorous and arsenic form p pi-p pi bonds with themselves and with other elements?
Asked by deepti_walvekar | 22 Oct, 2010, 12:00: AM
Phosphorous has electronic configuration 1s2,2s2,2p6,3s2,3p3,3d0 and only three 'p 'orbitals can participate during the formation of the bond and one 'p' orbital will form one sigma bond and other will formed pie bond beacuse of the positions of 'p' orbitals in the space which is not allowing them to make sigma bond and they are only able to make pie bond.
Answered by | 22 Oct, 2010, 11:40: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by gurugubellisaivishal2705 | 09 Jul, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by nonuhasan2 | 20 Nov, 2021, 12:05: PM
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 03:49: PM
CBSE 12-science - Chemistry
Asked by cute44464 | 01 Mar, 2021, 01:17: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 10:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 11:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 01:21: PM
CBSE 12-science - Chemistry
Asked by nishaohlyan835 | 30 Jun, 2020, 04:18: PM