CBSE Class 10 Answered
Why are certain compunds called hydrocarbons.write the general formula for homologus series of alkanes,alkenes and alkynes. And draw the ele tron dot formula for tne first member of each.
Asked by aahlad06 | 10 Jan, 2018, 09:21: PM
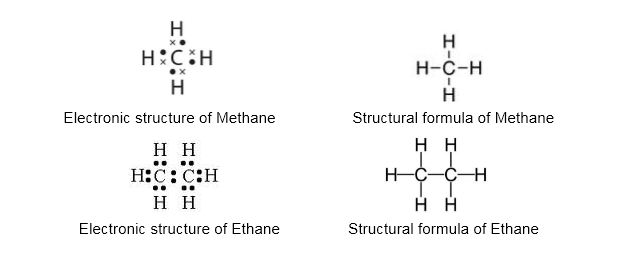
Long chain carbon compounds containing a backbone of carbon atoms and associated or bonded with hydrogen atoms are called hydrocarbons.
Alkanes:
- Alkanes are hydrocarbons in which all the linkages between the carbon atoms are single covalent bonds.
- General formula of alkanes: CnH2n+2
Alkenes:
- Alkenes are unsaturated aliphatic hydrocarbons containing a carbon-carbon double bond.
- General formula of alkenes: CnH2n
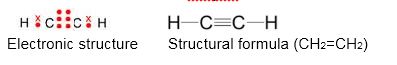
Alkynes:
- Alkynes are unsaturated aliphatic hydrocarbons containing a carbon-carbon triple bond in their molecule.
- The general formula of alkynes is CnH2n−2.
Answered by Ramandeep | 11 Jan, 2018, 11:42: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM
CBSE 10 - Chemistry
Asked by sunitha4503 | 28 Jul, 2020, 10:25: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:41: PM