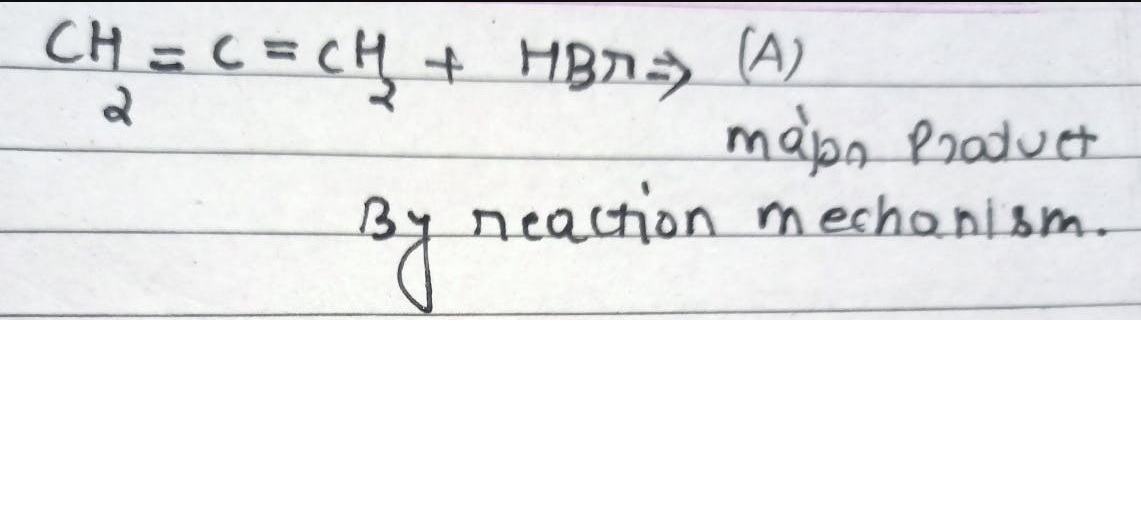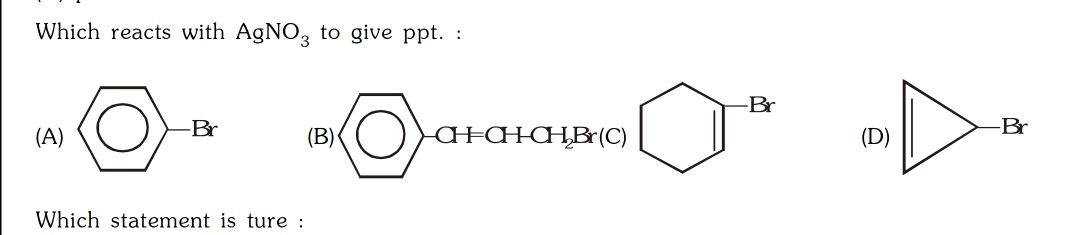CBSE Class 12-science Answered
why are arylhalide less reactive than alkyl halide in nucleophilic substitution reaction?
Asked by medha_sharma | 30 Nov, 2014, 10:20: AM
Dear shankerabhi@yahoo.co.in
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
The carbon-chlorine bond in chlorobenzene is stronger than expected. There is an interaction between one of the lone pairs on the chlorine atom and the delocalised ring electrons, and this strengthens the bond. Hence there is an extra strength of the carbon-halogen bond in aryl halides.
Both Sn1 and SN2 mechanisms involve breaking the carbon-halogen bond at some stage. It is difficult to break this bond. Hence, nucleophillic reactions are difficult in aryl halides. This is not the case in alkyl halides.Regards
Topperlearning Team.
Answered by Prachi Sawant | 01 Dec, 2014, 12:43: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM