CBSE Class 12-science Answered
Why anti-markovnikov rule is app;ied only with HBr?How does FeCl3 act as a halogen carrier?
Asked by Kishujaiswal353 | 06 Sep, 2014, 05:44: PM
Dear Kishujaiswal353@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We cannot entertain 2 questions in a single query. In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Regards
Topperlearning Team.
Answer to your first question is given below:
Why anti-markovnikov rule is applied only with HBr?
- The peroxide effect is not observed in case of H-Cl and H-I.
- This is because H-Cl bond (bond enthalpy 430.5kJmol-1) is stronger than H-Br bond (bond enthalpy 367.7 kJmol-1) and not broken by free radical while H-I bond (bond enthalpy 363.7 Kjmol-1) is weaker and iodine free radicals combine together to form iodine molecule instead of adding to double bond.
Answered by Arvind Diwale | 07 Sep, 2014, 01:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




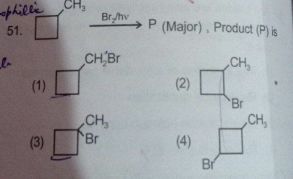
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-