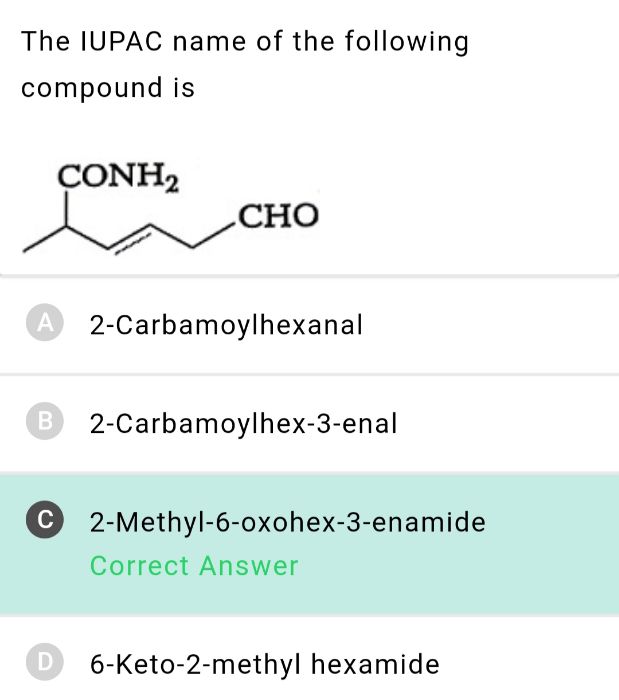
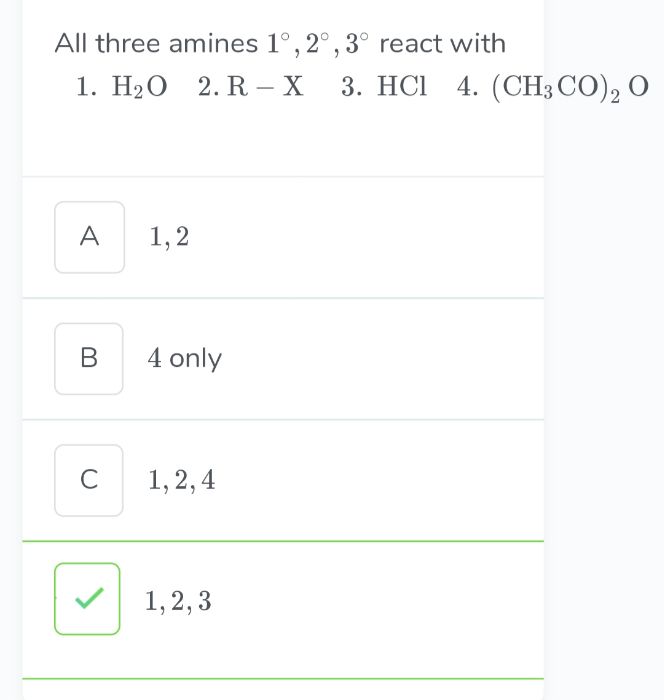
CBSE Class 12-science Answered
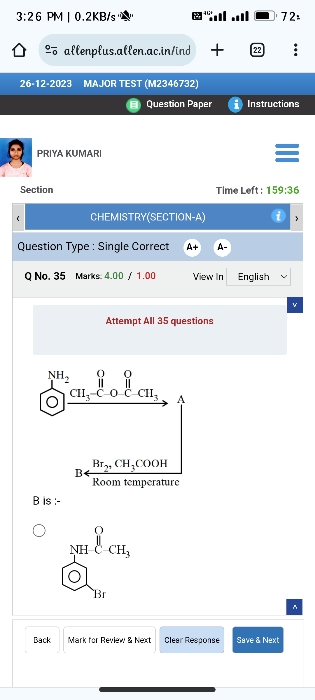
Why aniline readily reacts with bromine to give tri bromo derivative?
Asked by piyush das | 28 Aug, 2011, 12:00: AM

addition of bromine to aniline in an electrophilic addition.When the electrophile attacks the ortho and para positions of aniline, the nitrogen atom can donate electron density to the pi system giving four resonance structures This substantially enhances the stability of the cationic intermediate. Here A is representing Br+
Answered by | 29 Aug, 2011, 04:54: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by priyankapaliwal255 | 23 Sep, 2023, 05:46: AM
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 07 Jul, 2022, 08:13: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by dhivagar25375 | 12 Aug, 2020, 08:34: PM
CBSE 12-science - Chemistry
Asked by danapalanandhan | 28 Jul, 2020, 11:48: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 27 May, 2020, 03:34: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM