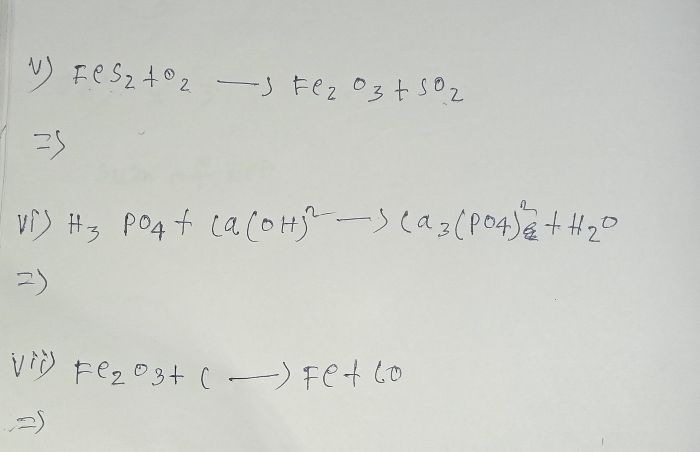CBSE Class 10 Answered
Which of the following reactions will produce more volatile acid:
Pb(NO3)2 + H2SO4(dil.) --> PbSO4 + 2HNO3
KNO3 + H2SO4(conc.) --> KHSO4 + HNO3
Asked by arpitt682 | 05 Jan, 2020, 11:21: AM
Dilution increases volatality. So when concentration increases volatality decreases.
So dilute H2SO4 will prodeuce more volatile acid.
Answered by Ravi | 07 Jan, 2020, 11:08: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM