CBSE Class 11-science Answered
Which is the element that has the highest first ionization potential?
Asked by Trisha Gupta | 20 Feb, 2020, 08:47: PM
Ionisation potential increases across a period and decreases down a group. Helium (He) is having highest first ionization potential(2370 KJ mol-1)
Answered by Ramandeep | 24 Feb, 2020, 03:15: PM
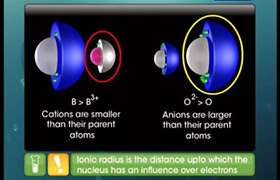
Concept Videos
CBSE 11-science - Chemistry
Asked by dreamaqua2017 | 04 Feb, 2024, 04:25: PM
CBSE 11-science - Chemistry
Asked by kamyabansal2905 | 16 Jul, 2022, 07:11: PM
CBSE 11-science - Chemistry
Asked by jigneshd5127 | 18 Jan, 2021, 09:16: AM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 30 May, 2020, 11:30: AM
CBSE 11-science - Chemistry
Asked by ayushvasava2003 | 09 Mar, 2020, 02:28: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 20 Feb, 2020, 08:47: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 19 Feb, 2020, 08:54: PM
CBSE 11-science - Chemistry
Asked by amulyagampa2002 | 12 Dec, 2018, 11:29: AM
CBSE 11-science - Chemistry
Asked by Ujwalsidhu19 | 08 Nov, 2018, 12:43: PM