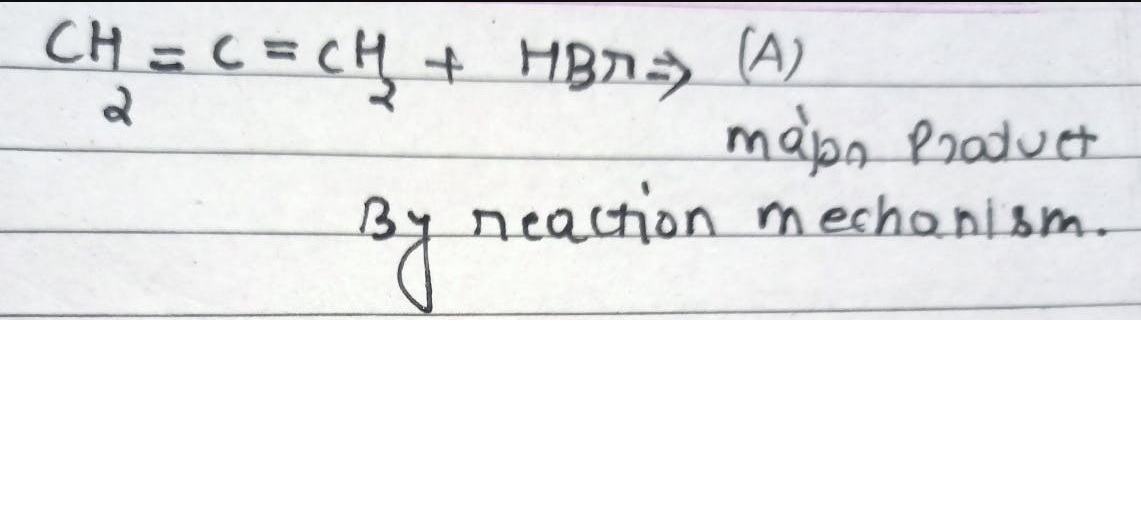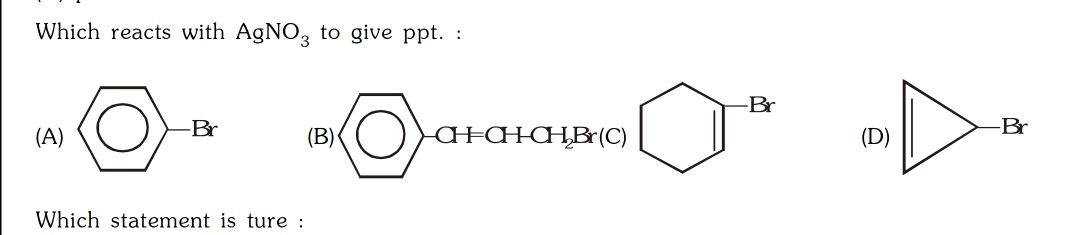CBSE Class 12-science Answered
Which is a better nucleophile - iodine ion or a bromine ion? Why?
Asked by aditichobey | 31 May, 2014, 03:47: PM
- Nucleophilicity increases as we go down the periodic table.
- So iodide ion is a better nucleophile than bromide ion because iodine is one row down from bromine on the periodic table.
Answered by Vaibhav Chavan | 02 Jun, 2014, 12:32: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM