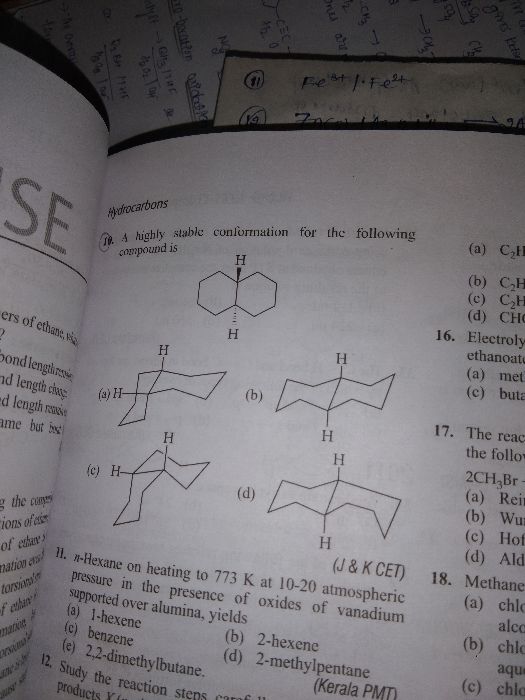
CBSE Class 11-science Answered
where is the nodal plane of pi bond of ethene located ? how is it located there?
Asked by gnikhilvarma | 31 May, 2009, 06:31: PM
In ethene, there are five sigma bonds in one plane: one C-C sigma bond and four C-H sigma bonds.
The pi bond is formed perpendicular to the plane in which one lobe of electron density is above the plane and one lobe of electron density is below the plane.
The nodal plane of the pi bond is in between the two lobes of electron density of the pi bond.
Answered by | 12 Jun, 2009, 05:02: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM