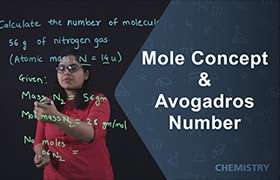CBSE Class 9 Answered
When we use this formula no.of moles =given of particles/Avogadro no.
Also give me one ex. of this.
When we use this formula no.of moles= given mass/molar mass* Avogadro no.
Also give me one ex. of this.
How i can know what formula is applicable in the exam??????
tell me this clearly becoz exams are coming near and near?and this thing is not clear to me...
Asked by | 05 Dec, 2012, 12:50: PM
Here is a graphic of the procedure steps:
1. no.of moles =given no of particles/Avogadro no.
Problem #1: 0.200 mole of H2O contains how many molecules?
Solution: no.of moles =given no of particles/Avogadro no.
given no of particles = no.of moles x Avogadro no.
0.200 mol x 6.022 x 1023 mol¯1
2. moles = mass ÷ molar mass
Calculate the moles in 124.5 g of oxygen gas.
- Write the equation:
n = m ÷ M - Extract the data from the question:
m = 124.5 g - Calculate the molar mass of the substance using the periodic table:
M(O2) = 2 x 16.00 =32.00 g mol-1 - Substitute the values into the equation and solve:
n = 124.5 ÷ 32.00 = 3.89 mol
Answered by | 05 Dec, 2012, 01:58: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM