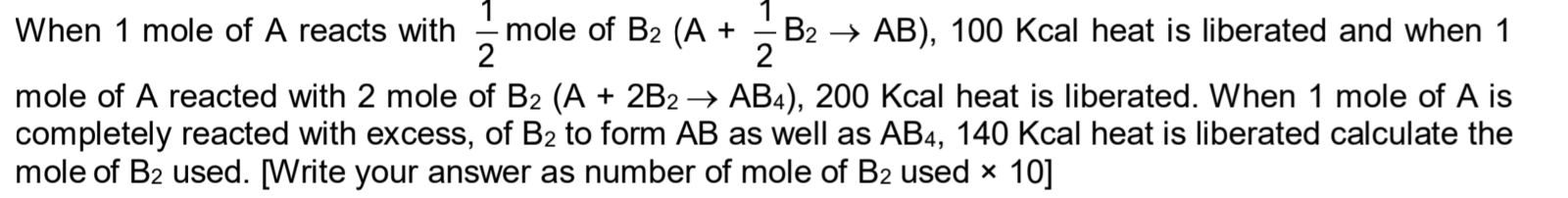CBSE Class 11-science Answered
When 400 g of a 20% solution by weight was cooled, 50 g of solute precipitated. The percentage concentration of remaining solution is
Asked by Anil | 17 May, 2017, 03:07: PM
Wt. of solution = 400g
Wt. of solute = 20 x 400/100 = 80 g
Wt. of solute precipitated = 50g
Wt. of solute remained in solution = 80-50 = 30g
Wt of solution remained = 400 – 50 = 350g
Hence % by weight = 30/350x 100 = 8.57%
Answered by Vaibhav Chavan | 17 May, 2017, 03:21: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM