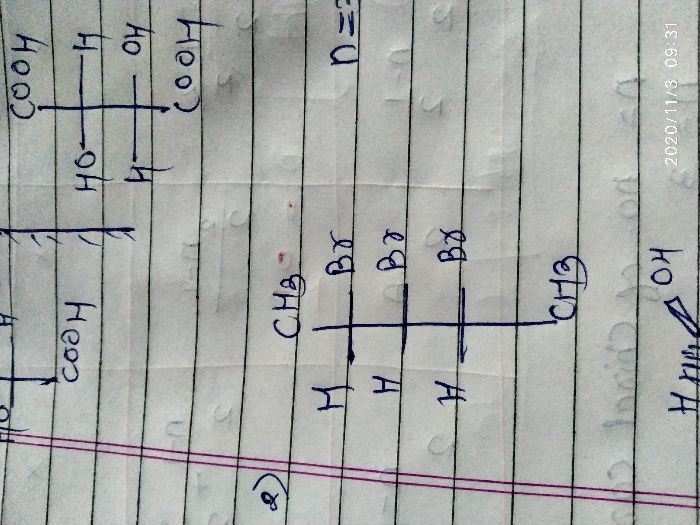CBSE Class 11-science Answered
whats the difference between hypercojugation and resononce ?
Asked by | 06 Mar, 2008, 03:02: PM
Hyperconjugation is delocalisation of sigma electrons of C-H attached directly to unsaturated carbon atom with an unshared p electrons and it is a permanent effect and stabilisation of cation depends on the number of alkyl groups attached to positive carbon or carb cation.Resonance is delocalisation of pi electons and so the carbon does not show properties of C=C or C-C bond.The polarity produced is +ve or -ve.
Answered by | 20 Dec, 2017, 04:55: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM
CBSE 11-science - Chemistry
Asked by rohus442 | 03 Nov, 2020, 09:31: AM
CBSE 11-science - Chemistry
Asked by ashok.amireddi | 01 May, 2020, 10:33: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 13 Apr, 2020, 08:36: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 12 Dec, 2019, 12:00: AM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 02 Sep, 2019, 02:26: PM
CBSE 11-science - Chemistry
Asked by musira29rahman | 30 Aug, 2019, 05:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Aug, 2019, 06:33: PM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 26 Aug, 2019, 09:39: PM