CBSE Class 12-science Answered
WHAT VOLUME OF 95 % SULPHURIC ACID ( DENSITY = 1.85 g cm^-3 ) AND WHAT MASS OF WATER MUST BE TAKEN TO PREPARE 100 cm^3 OF 15 % SOLUTION OF SULPHURIC ACID ( DENSTIY = 1.10 g cm^-3)?
Asked by kandappan | 02 Apr, 2020, 08:07: PM
To find:
Volume of 95% sulphuric acid (density = 1.85 g/mL)
Mass of water to prepare 100 mL of 15% solution of sulphuric acid (density = 1.10 g/mL)
Solution:
Volume of the solution = 100 cm2
Density of the solution = 1.10 g/cm3
Therefore, Mass of 100 cm3 of solution = 100 x 1.10 g =110 g
The given solutions is 15%. Therefore, 100 g of solution contains 15 g of H2SO4
Then, mass H2SO4 in 110 g (100 cm3 ) of solution = 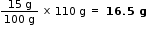
Mass of water in 110 g (100cm3 ) of solution = (110 - 16.5) g = 93.5 g
To obtain 100 cm3 of 15 % solution acid, we require the given information.
Mass of water = 93.5 g
Mass of H2SO4 (100% pure) = 16.5 g
Since, the given sulphuric acid is 95% pure, hence,
Mass of H2SO4 (95%) will be required =
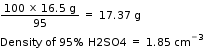
Volume of the solution = 100 cm2
Density of the solution = 1.10 g/cm3
Therefore, Mass of 100 cm3 of solution = 100 x 1.10 g =110 g
The given solutions is 15%. Therefore, 100 g of solution contains 15 g of H2SO4
Then, mass H2SO4 in 110 g (100 cm3 ) of solution = 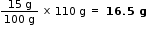
Mass of water in 110 g (100cm3 ) of solution = (110 - 16.5) g = 93.5 g
To obtain 100 cm3 of 15 % solution acid, we require the given information.Mass of water = 93.5 g
Mass of H2SO4 (100% pure) = 16.5 g
Since, the given sulphuric acid is 95% pure, hence,
Mass of H2SO4 (95%) will be required =
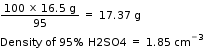
Answered by Ramandeep | 03 Apr, 2020, 11:03: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM








