CBSE Class 11-science Answered
What volume of .1M Ca (OH)2 is sufficient to neutralise 100 ml solution containing .1N HCl and .1N H2SO4
Asked by vishakhachandan026 | 19 Apr, 2019, 10:16: AM
In case neutralisation
Mili equivalents of base = Mili equivalents of acid
So,
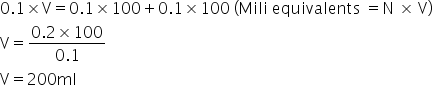
Answered by Ravi | 19 Apr, 2019, 10:43: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







