CBSE Class 12-science Answered
What is thhe relation between coordination compounds and molar conductivity.
Asked by ankit malhotra | 12 May, 2014, 07:19: AM
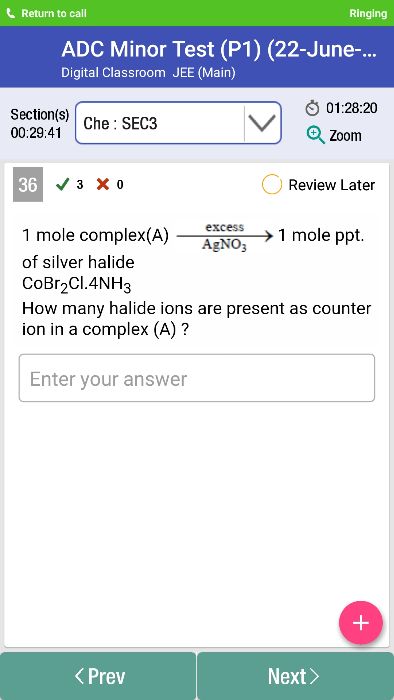
- The measurement of molar conductances (Lm) of solutions of coordination compounds helps to estimate the number of the ions furnishes by the compound in solution.
- By comparing the molar conductance of the compound with those of known electrolytes, it was able to predict the number of ions present in the solution.
For example:
It has been observed that the complex CoCl3.6NH3 behaved as 1:3 electrolyte, CoCl3.5NH3 as 1:2 electrolyte, CoCl3.4NH3 as 1:1 electrolyte.
The coordination compound CoCl3.3NH3 behaved as molecular (non-electrolyte).
Answered by Vaibhav Chavan | 12 May, 2014, 11:06: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by prathyushagn1 | 09 Dec, 2020, 08:12: AM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 31 Aug, 2020, 08:24: PM
CBSE 12-science - Chemistry
Asked by sha.bijoy17 | 07 Aug, 2020, 11:55: AM
CBSE 12-science - Chemistry
Asked by Shambhuhd79 | 22 Jun, 2020, 11:09: AM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Feb, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by smit230503 | 04 Feb, 2020, 08:56: PM
CBSE 12-science - Chemistry
Asked by monishadubey202 | 08 Jan, 2020, 03:42: PM
CBSE 12-science - Chemistry
Asked by Chakshu29saini | 17 Sep, 2019, 06:19: PM
CBSE 12-science - Chemistry
Asked by bjayanta | 24 Mar, 2019, 08:56: PM
CBSE 12-science - Chemistry
Asked by himanshuneb | 28 Jan, 2019, 10:33: PM