CBSE Class 12-science Answered
What is the shortest wave length in Paschen series of hydrogen spectrum.
A) 8206 angstrom units
B) 6208 angstrom units
C) 5494 angstorm units
D) 4945 angstorm units
Pls explain the answer & tell me the concept !!!!
Asked by GeoffreyRichards | 23 Nov, 2010, 05:50: PM
Rydberg formula
The energy differences between levels in the Bohr model, and hence the wavelengths of emitted/absorbed photons, is given by the Rydberg formula
where n is the initial energy level, n′ is the final energy level, and R is the Rydberg constant
For Paschen series : n' = 3
therefore
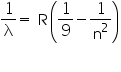
for shortest wavelengh (λs ) 1/λs which means n=∞ in above equation
therefore
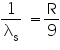
which gives λs =8206 angstrom
Answered by | 24 Nov, 2010, 11:26: AM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by Akshatkuma2004 | 04 Jul, 2021, 04:04: PM
CBSE 12-science - Physics
Asked by kbsahilsid18 | 13 Aug, 2020, 10:02: AM
CBSE 12-science - Physics
Asked by rsrakesh932 | 12 Jun, 2020, 04:38: PM
CBSE 12-science - Physics
Asked by jabreshwar1008 | 28 Dec, 2019, 08:59: PM
CBSE 12-science - Physics
Asked by yadavnitish688 | 14 Dec, 2019, 06:04: AM
CBSE 12-science - Physics
Asked by sidiz.shrestha07 | 26 May, 2019, 06:42: AM
CBSE 12-science - Physics
Asked by pardeepkumar2281 | 28 Feb, 2019, 07:24: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:54: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:51: PM


