NEET Class neet Answered
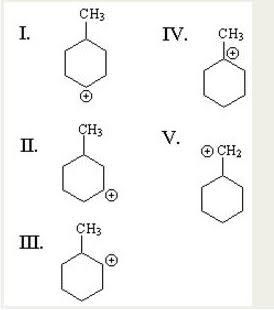
Carbocations are classified as primary, secondary, or tertiary depending on the number of carbon atoms bonded to the ionized carbon. Primary carbocations have one or zero carbons attached to the ionized carbon, secondary carbocations have two carbons attached to the ionized carbon, and tertiary carbocations have three carbons attached to the ionized carbon.
Stability of the carbocation increases with the number of alkyl groups bonded to the charge-bearing carbon. Tertiary carbocations are more stable than secondary carbocations; primary carbocations are highly unstable because, while ionized higher-order carbons are stabilized by hyperconjugation, unsubstituted (primary) carbons are not.
For Carbocations: the stability order is
TERTIARY > SECONDARY > PRIMARY > PRIMARY
(With alkyl group) (Without alkyl group)
The stability order of carbocations bearing only alkyl groups is:
30> 20> 10> CH3.
Carbocation stability is influenced by three factors:
a) Hyperconjugation- Increasing the number of alkyl substituents increases the stability of the carbocation. This is due to orbital overlap between the ?-bond and the empty p orbital on the sp2 carbon.
b) Inductive Effects- Neighboring alkyl groups contain electrons that are polarizable, and these can shift towards the positive charge. (Small Hydrogen substituents cannot do this as well).
c) Resonance Effects- Conjugation with a multiple bond or lone pairs of electrons increases the stability of a carbocation.