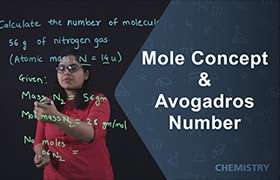CBSE Class 9 Answered
What is the no. of molecules in a drop of water weighing 0.05g.?
Asked by ananya vyas | 08 Feb, 2011, 10:12: AM
Dear Student
One drop of water has the weight = 0.05 g.
The molar mass of water (H2O) is 18.0 grams/mol (1.008 + 1.008 + 16.0).
This means there is one mole of water in 18.0 grams. One mole is 6.02 × 1023 molecules.
Then the number of atoms in 0.5 g:
0.05 grams ÷ 18.0 grams × (6.02 × 1023 molecules)= 1.67 × 1022 molecules
Then the number of atoms in 0.5 g:
0.05 grams ÷ 18.0 grams × (6.02 × 1023 molecules)= 1.67 × 1022 molecules
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 09 Feb, 2011, 12:25: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM