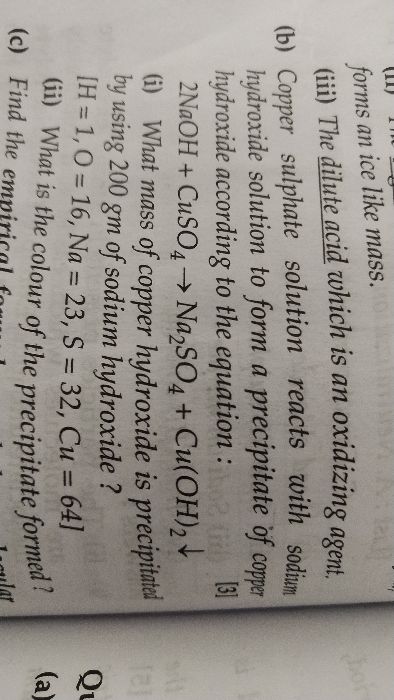ICSE Class 10 Answered
What is the mass of calcium carbonate precipitated when calcium chloride solution is added to a solution containing 21.2g of sodium carbonate?
RMM of sodium carbonate = 106
RMM of calcium carbonate = 100
Asked by ben_kpg | 20 Jun, 2019, 04:12: PM
NaCO3 + CaCl2 → NaCl + CaCO3
21.2g
The relative molar mass of NaCO3 is 106 g per mole.
This means mass of 1 mole of NaCO3 = 106 g
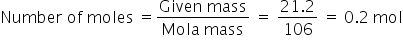
1 mole of NaCO3 liberates 1 mole of CaCO3
Therefore 0.2 mole of NaCO3 liberates 0.2 mole of CaCO3
= 100×0.2 .......................... (relative molar mass of CaCO3=100)
= 20g
Answered by Ramandeep | 21 Jun, 2019, 02:07: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM