CBSE Class 9 Answered
What is the electronic configuration of isotopes of chlorine and isobars of calcium and argon?
Asked by Topperlearning User | 16 Mar, 2015, 05:24: PM
Pair of isotopes: 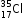 and
and 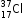
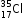 and
and 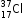
Electronic configuration is 2, 8, 7 because both the atoms have the same atomic number and the same number of electrons as 17.
Pair of isobars:  and
and 
Electronic configuration of calcium (Ca) with atomic number 20 is 2, 8, 8, 2. Electronic configuration of argon (Ar) with atomic number 18 is 2, 8, 8.
 and
and 
Electronic configuration of calcium (Ca) with atomic number 20 is 2, 8, 8, 2. Electronic configuration of argon (Ar) with atomic number 18 is 2, 8, 8.
Answered by | 16 Mar, 2015, 07:24: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namyairmalwar9803.5sdatl | 07 Jun, 2020, 01:47: PM
CBSE 9 - Chemistry
Asked by pradoshsanesar | 10 Jan, 2020, 12:01: PM
CBSE 9 - Chemistry
Asked by maltidevi8800 | 06 Dec, 2019, 09:29: PM
CBSE 9 - Chemistry
Asked by ABHILASHA | 22 Aug, 2019, 08:46: AM
CBSE 9 - Chemistry
Asked by dubeykrishna49025 | 14 Oct, 2018, 10:02: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:05: PM









