CBSE Class 12-science Answered
what is the differrence between anthracene and phenanhrene? and how is phenanthrene more stable than anthracene? it is out of topipc but please answer.
Asked by saket shandilya | 22 May, 2014, 12:08: PM
Both anthracene and phenanthrene are polycyclic aromatic hydrocarbon having fused benzene rings.
Anthracene
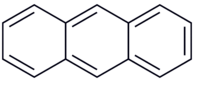
Phenanthrene
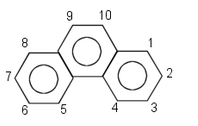
Phenanthrene is stable than anthracene due to more efficient π bonding and not because of hydrogen bonding. In phenanthrene, one resonance structure has two sextets (benzene-like moieties) at the extremities, while the other resonance structure has just one central sextet. Therefore in this molecule the outer rings are firmly aromatic while its central ring is less aromatic and therefore more reactive. In contrast, in anthracene, the number of sextets is just one and aromaticity spreads out.
Answered by Prachi Sawant | 26 May, 2014, 11:09: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by jaiadithya05 | 20 Sep, 2021, 07:20: PM
CBSE 12-science - Chemistry
Asked by shantasharma611 | 01 May, 2021, 01:51: PM
CBSE 12-science - Chemistry
Asked by sivaveeramachaneni9 | 08 Feb, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by kalkikai33 | 01 Jun, 2020, 03:40: PM
CBSE 12-science - Chemistry
Asked by subhasmitaswainstudent | 02 May, 2020, 01:22: PM
CBSE 12-science - Chemistry
Asked by buluacharya123 | 25 Apr, 2020, 11:37: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 10:58: PM
CBSE 12-science - Chemistry
Asked by tn6380313887.mohanviji | 09 Mar, 2020, 09:19: AM
CBSE 12-science - Chemistry
Asked by nidhi.jain0212 | 07 Mar, 2020, 01:30: PM