ICSE Class 10 Answered
what is the difference between thermal dissociation and thermal decomposition?
Asked by Vandana | 14 Jun, 2017, 07:57: PM
Any process of decomposition by heat is called thermal decomposition. A thermal decomposition may be reversible or irreversible reaction.
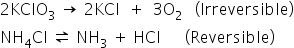
Reversible thermal decomposition reactions, i.e. in which the prodcuts of decomposition can re-unite to form the original substance when the agent (heat) which brings about the decomposition is withdrawn are called thermal dissociation.
Thus, thermal dissociation is a special case of thermal decomposition.
Answered by Prachi Sawant | 15 Jun, 2017, 10:58: AM
Concept Videos
ICSE 10 - Chemistry
Asked by tk5363508 | 01 Apr, 2024, 08:36: PM
ICSE 10 - Chemistry
Asked by dhakankar | 25 Oct, 2023, 11:07: AM
ICSE 10 - Chemistry
Asked by suhani.goyal.brps | 19 Oct, 2020, 08:17: PM
ICSE 10 - Chemistry
Asked by praptithombre47.10spicertl | 13 Jun, 2020, 01:09: PM
ICSE 10 - Chemistry
Asked by kartikmangaliya22.10spicertl | 27 May, 2020, 10:01: PM
ICSE 10 - Chemistry
Asked by vsejwani53 | 01 Jan, 2020, 12:23: PM
ICSE 10 - Chemistry
Asked by pradyumna2008civil | 10 Nov, 2019, 12:05: PM
ICSE 10 - Chemistry
Asked by mamtapramod1976 | 27 Sep, 2019, 06:19: PM
ICSE 10 - Chemistry
Asked by pradipdhole | 09 Jun, 2019, 11:03: PM
ICSE 10 - Chemistry
Asked by shreya.saraf.2005 | 15 Feb, 2019, 03:30: PM




