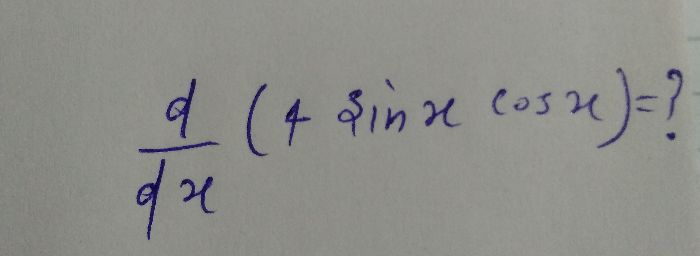NEET Class neet Answered
What is Mol
Asked by sandeepias70030l | 30 May, 2022, 09:01: PM
Mole ( symbol is mol) is the unit used by chemists and scientists to measure the amount of substance.
If the substance is element , then one mole of substance is one gram atomic weight , i.e., atomic weight expressed in gram
For example 1 mole of carbon is 12 gram because atomic weight of carbon is 12 atomic mass unit.
For example 1 mole of Calcium is 40.08 gram because atomic weight of calcium is 40.08 atomic mass unit.
-----------------------
If the substance is compound , then one mole of substance is one gram moleculer weight , i.e., moleculer weight expressed in gram
For example 1 mole of Sodium chloride (NaCl) is 58.44 gram because moleculer weight of NaCl is 58.44 atomic mass unit.
For example 1 mole of Calcium Carbonate (CaCO3) is 100.09 gram because moleculer weight of CaCO3 is 100.09 atomic mass unit.
If the substance is element , then one mole of substance has 6.022 × 1023 atoms , where this number 6.022 × 1023 is called as Avagadro number.
If the substance is compound , then one mole of substance has 6.022 × 1023 molecules .
Answered by Thiyagarajan K | 30 May, 2022, 11:03: PM
NEET neet - Physics
Asked by hindujaguttula890 | 29 Mar, 2024, 08:34: AM
NEET neet - Physics
Asked by sadiyakhan54325432 | 18 Oct, 2023, 09:44: PM
NEET neet - Physics
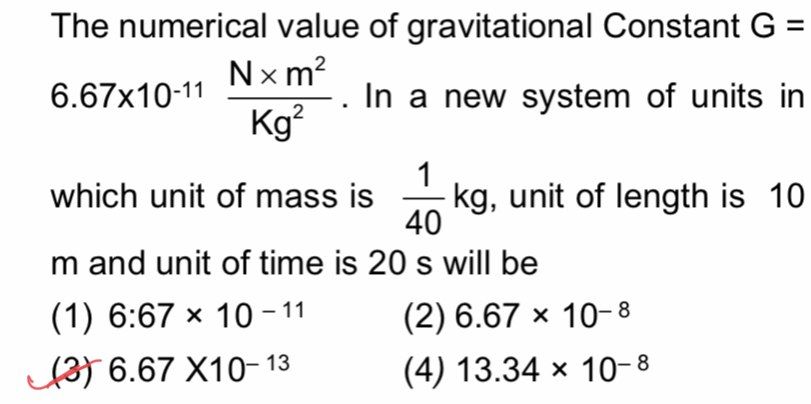
Asked by sandippatange840 | 10 Apr, 2023, 09:30: AM
NEET neet - Physics
Asked by jantikaromprakash | 18 Aug, 2022, 12:37: PM
NEET neet - Physics
Asked by sandeepias70030l | 30 May, 2022, 09:01: PM
NEET neet - Physics
Asked by gurjarhariomgurjar605 | 19 May, 2022, 10:40: PM
NEET neet - Physics
Asked by surabhilakhara7299 | 30 Jan, 2022, 02:16: PM
NEET neet - Physics
Asked by surabhilakhara7299 | 20 Jan, 2022, 10:51: PM
NEET neet - Physics
Asked by jhajuhi19 | 11 Dec, 2021, 05:44: PM
NEET neet - Physics
Asked by pricemela2 | 07 Oct, 2021, 04:17: PM