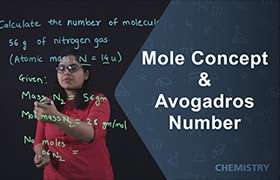CBSE Class 9 Answered
What is law of multiple proportion?
Asked by | 20 Nov, 2012, 04:36: PM
The law of multiple proportions states that if two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers. This law refers to different chemical compounds that can be formed when two elements react with each other. For example, hydrogen and oxygen can react to form water (H2O) and hydrogen peroxide (H2O2). In the first compound, one gram of hydrogen combines with 8 grams of oxygen. In the second, one gram of hydrogen reacts with 16 grams of oxygen. If we look at the ratio of these to each other, 8/16 =2, which is a whole number.
Answered by | 20 Nov, 2012, 04:45: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM