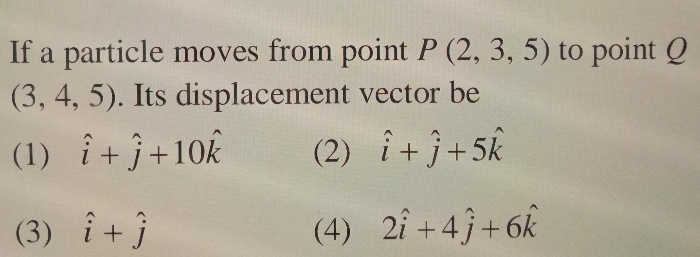CBSE Class 11-science Answered
what is kinetic theory
Asked by Tom Joe Joe | 12 Apr, 2011, 12:00: AM
Theory describing the physical properties of matter in terms of the behaviour – principally movement – of its component atoms or molecules. It states that all matter is made up of very small particles that are in constant motion, and can be used to explain the properties of solids, liquids, and gases, as well as changes of state. In a solid, the particles are arranged close together in a regular pattern and vibrate on the spot. In a liquid, the particles are still close together but in an irregular arrangement, and the particles are moving faster and are able to slide past one another. In a gas, the particles are far apart and moving rapidly, bouncing off the walls of their container. The temperature of a substance is dependent on the velocity of movement of its constituent particles, increased temperature being accompanied by increased movement.
Answered by | 12 Apr, 2011, 10:38: AM
Concept Videos
CBSE 11-science - Physics
Asked by om636694 | 04 Mar, 2024, 09:10: PM
CBSE 11-science - Physics
Asked by dhanapolla | 28 Jan, 2024, 10:40: AM
CBSE 11-science - Physics
Asked by santoshyadav6673633 | 26 Jan, 2024, 04:55: PM
CBSE 11-science - Physics
Asked by manjusrihalder395 | 07 Jan, 2024, 09:55: PM
CBSE 11-science - Physics
Asked by rahmanshah8572 | 29 Dec, 2023, 08:41: PM
CBSE 11-science - Physics
Asked by klvnsnthl | 25 Dec, 2023, 07:50: PM
CBSE 11-science - Physics
Asked by pothulasubbarayudu | 25 Dec, 2023, 06:58: PM
CBSE 11-science - Physics
Asked by neerajchaurasiya651 | 10 Dec, 2023, 09:32: PM
CBSE 11-science - Physics
Asked by debasishbarik2006 | 17 Nov, 2023, 07:03: PM