CBSE Class 12-science Answered
what is inert pair effect ?
Asked by sweety | 20 Oct, 2015, 08:17: PM
Inert pair effect: The tendency of the ns2 electron pair to participate in bond formation decreases with the increase in atomic size. Within a group, the higher oxidation state becomes less stable with respect to the lower oxidation state as the atomic number increases. This trend is called the inert pair effect. In other words, the energy required to unpair the ns2 electrons is more than the energy released in the formation of two additional bonds.
Answered by Vaibhav Chavan | 21 Oct, 2015, 08:19: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 17 Apr, 2020, 09:22: AM
CBSE 12-science - Chemistry
Asked by adarshkamble130 | 19 Aug, 2019, 12:22: AM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 10:50: PM
CBSE 12-science - Chemistry
Asked by ranvirsingh1as | 16 Dec, 2018, 10:47: PM
CBSE 12-science - Chemistry
Asked by Sneha | 16 Dec, 2018, 03:14: PM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:54: AM
CBSE 12-science - Chemistry
Asked by lekhakarthikeyan | 28 Aug, 2018, 03:52: AM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 01:00: AM




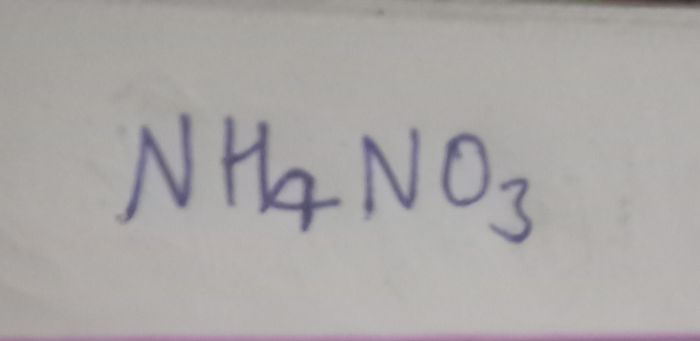
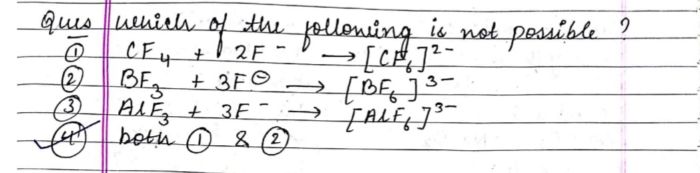
 to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc. 