CBSE Class 11-science Answered
What is a wave number ?Explain with full details along with the series in spectrum of hydrogen.
Asked by | 10 Jul, 2011, 12:00: AM
Wave number is defined as the number of wavelengths per centimetre and is equal to the inverse of wavelength expressed in centimetre. It is dented by 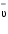 and is expressed in cm-1. A wavenumber can be converted into quantum-mechanical energy E in J or regular frequency ? in Hz according to
and is expressed in cm-1. A wavenumber can be converted into quantum-mechanical energy E in J or regular frequency ? in Hz according to
 ,
, .
.
Note that here wavenumber and the speed of light are in cgs units, so care must be taken when doing these calculations.
For example, the wavenumbers of the emissions lines of hydrogen atoms are given by
where R is the Rydberg constant and ni and nf are the principal quantum numbers of the initial and final levels, respectively (ni is greater than nf for emission).
Answered by | 11 Jul, 2011, 10:27: AM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 03:37: PM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 04:15: PM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 09:14: PM
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by advssdrall | 12 Jan, 2022, 05:12: AM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 22 Oct, 2021, 07:29: PM










