CBSE Class 12-science Answered
What happens when acetaldehyde reacts with (1) semicarbazide (2) HCN (3) I2/NaOH (4) NaHSo3 (5) Dil. NaoH (6) phenyl hydrazine
Asked by ap996969 | 16 Mar, 2019, 10:14: PM
when acetaldehyde reacts with
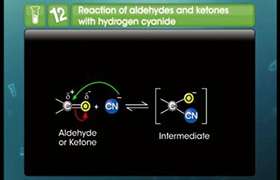
(2) HCN
Addition of HCN to aldehydes and ketones yieldscyanohydrins.
Because the reaction is very slow with pure HCN, it is catalysed with the help ofa base and the cyanide ion (CN−) generated as a strong nucleophile adds to carbonyl compounds to give cyanohydrins.
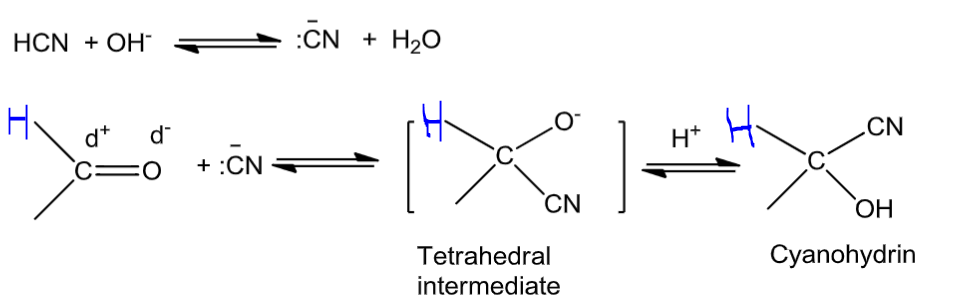
(3) I2/NaOH
Aldehydes and ketones with at least one methyl group attached to the carbonyl carbon atom on oxidation with sodium hypohalite turn to sodium salts of corresponding acids with one carbon atom less than that of the carbonyl compound.In this reaction, the methyl group is converted to haloform.
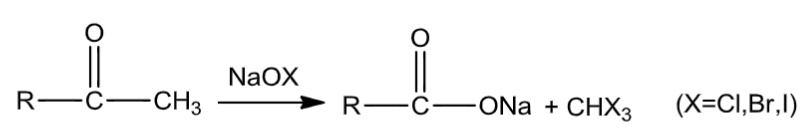
(4) NaHSO3:
Sodium hydrogen sulphite when added to aldehydes and ketones yields addition products.
Because of steric factors, the equilibrium is on the right-hand side for most aldehydes, and it is on the left-hand sidefor most ketones.
(5) Dil. NaoH
Aldehydes and ketones with at least one α-hydrogen undergo reaction in the presenceof dilute alkali as a catalystto formβ-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol),respectively. This is known as aldol reaction
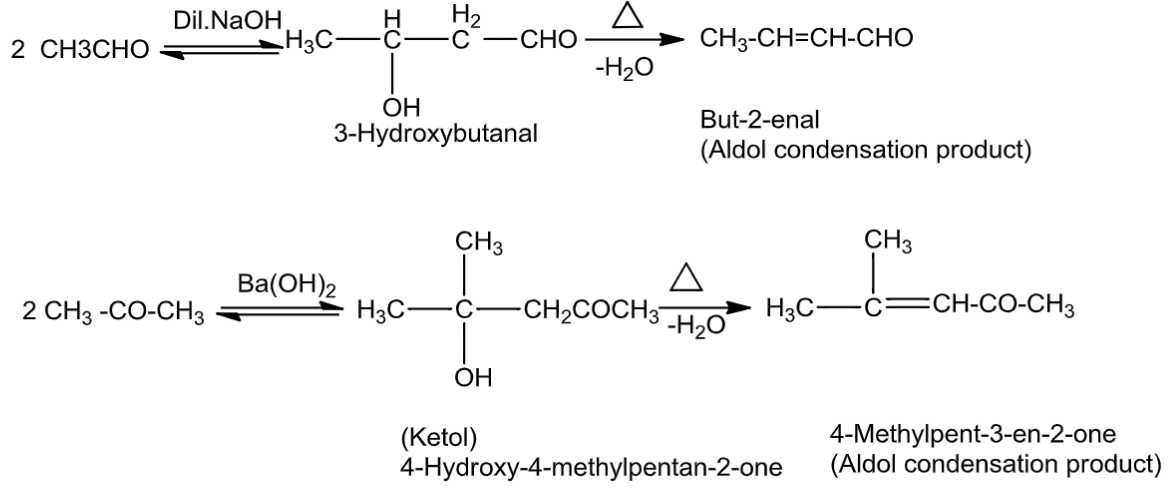
Answered by Ramandeep | 17 Mar, 2019, 11:54: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by ajayarchi | 08 Feb, 2024, 03:43: AM
CBSE 12-science - Chemistry
Asked by pallasriramulu9 | 24 Dec, 2023, 06:05: AM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 30 Jun, 2021, 04:52: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 28 Jun, 2021, 02:34: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 31 Dec, 2020, 10:45: AM
CBSE 12-science - Chemistry
Asked by shreevarshni1910 | 16 Jun, 2020, 01:36: PM
CBSE 12-science - Chemistry
Asked by yukthas706 | 11 Jun, 2020, 10:00: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 14 Feb, 2020, 12:09: PM
CBSE 12-science - Chemistry
Asked by Www.keshariramesh32 | 23 Nov, 2019, 02:02: PM