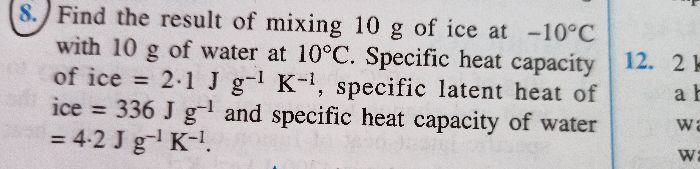ICSE Class 10 Answered
What happens when 500 grams of ice is added to 5 kilograms of water at 50 degree celcius.Take specific heat capacity of water = 4200 J/kg degree celcius and specific latent heat of fusion of ice = 336000J/kg ?
Asked by Satyanita - | 12 Apr, 2014, 02:16: PM
Let the final temperature be q.
Energy absorbed by 500 g of ice on melting at 0 °C is
Q = ml = 0.5 × 3.36 × 105 = 1.68 × 105 J
Q = ml = 0.5 × 3.36 × 105 = 1.68 × 105 J
Energy lost by 5 kg of water in cooling from 50 °C to q is
Q = 5 × 4200 × (50 - q) = 21000 (50 - q)
Q = 5 × 4200 × (50 - q) = 21000 (50 - q)
Heat gained = heat lost
1.68 × 105 J = 21000 (50 - q)
0.8 = 50 - q
or, q = 50 - 0.8 = 49.2oC
Thus, when 500 grams of ice is added to 5 kilograms of water at 50 degree celcius, the final temperature attained by the mixture is 49.2oC.
Answered by | 14 Apr, 2014, 12:47: AM
Concept Videos
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 02:35: PM
ICSE 10 - Physics
Asked by surendrakumarclw | 20 Mar, 2021, 06:16: PM
ICSE 10 - Physics
Asked by anuradhastudent12 | 27 Feb, 2021, 08:33: PM
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by charan3802 | 23 Oct, 2020, 10:29: AM
ICSE 10 - Physics
Asked by khushalkumarsk | 17 Aug, 2020, 12:37: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by abhinabasarkar.abhinaba | 17 Jun, 2020, 09:24: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 03:00: PM
ICSE 10 - Physics
Asked by chaitalisen977 | 19 May, 2020, 03:32: PM