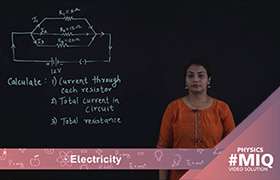
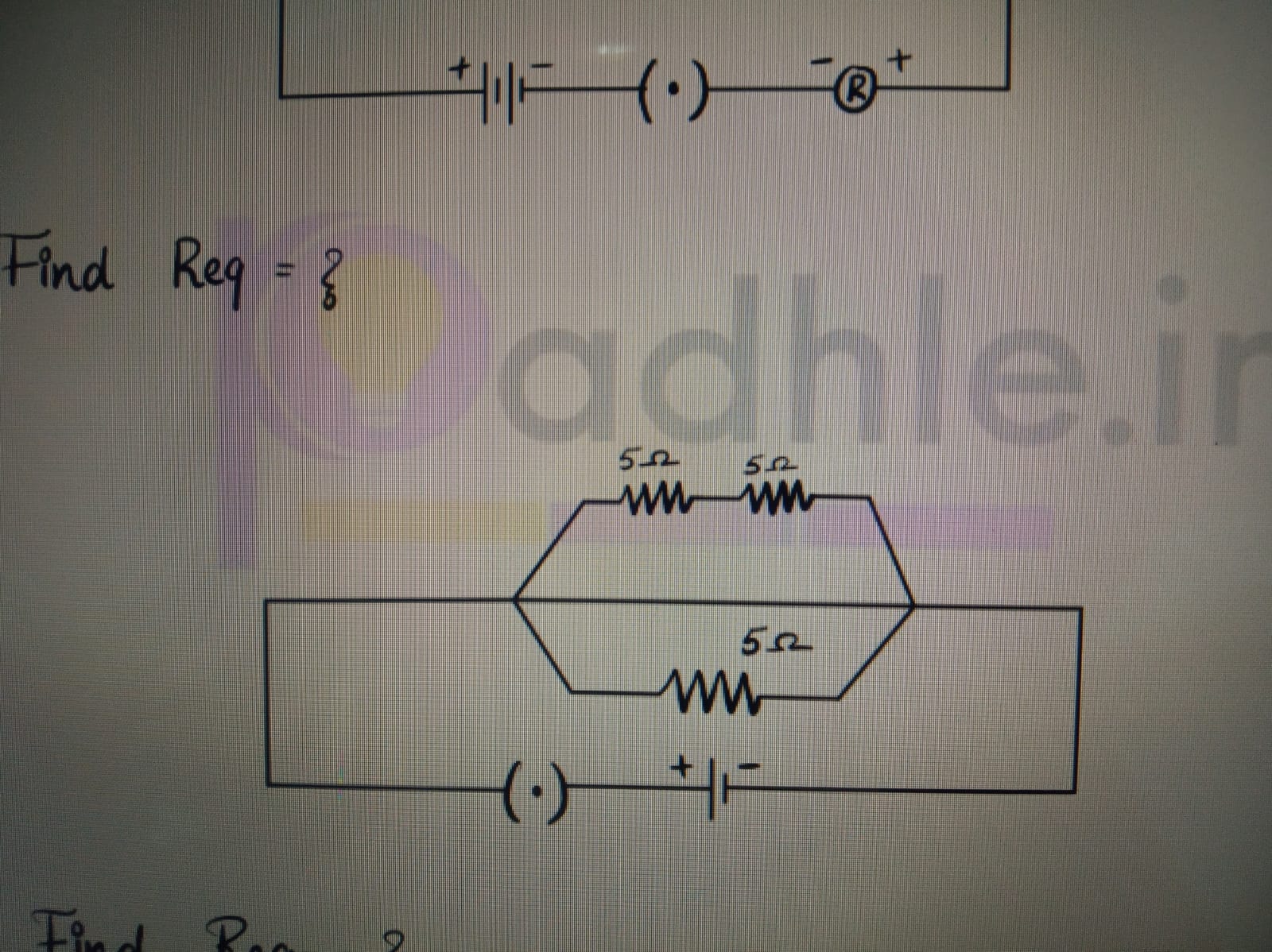
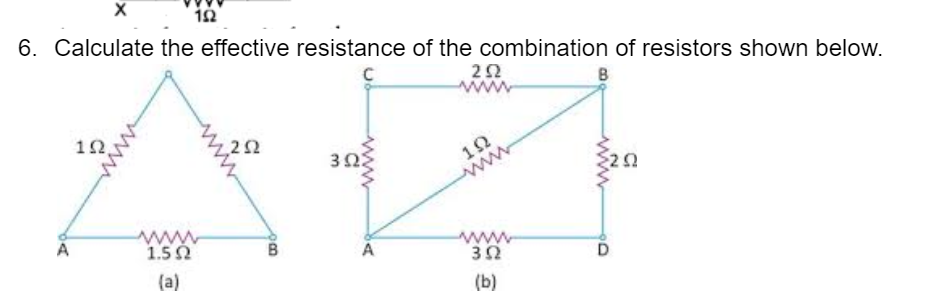
CBSE Class 10 Answered
|
VOLTA'S FIRST BATTERY
|
The battery made by Volta is credited as the first electrochemical cell. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte is sulfuric acid or a brine mixture of salt and water. The electrolyte exists in the form 2H+ and SO42-. The zinc, which is higher than both copper and hydrogen in the electrochemical series, reacts with the negatively charged sulfate SO42- . The positively charged hydrogen ions (protons) capture electrons from the copper, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode. We now have two terminals, and the current will flow if we connect them. The reactions in this cell are as follows: zinc Zn --> Zn2+ + 2e- sulfuric acid 2H+ + 2e- --> H2 The copper does not react, functioning as an electrode for the chemical reaction. |