CBSE Class 12-science Answered
What causes hardness of water? Why soap fails to work in hard water?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
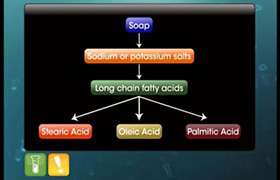
Hardness of water is due to the presence of calcium and magnesium salts. These ions form insoluble calcium and magnesium soaps when sodium or potassium soaps are dissolved in water. The insoluble soap separates as scum in water and are useless as cleansing agents.


Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 02:14: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:50: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 02:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:52: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 02:20: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 04:54: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 04:56: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jul, 2014, 05:03: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:52: AM