JEE Class main Answered
What are the formula to calculate distance between nearest neighbor in different types of solids and what is the relationship between distance of nearest neighbor and radius of cation and anion ?
Asked by inbasri224 | 23 Feb, 2019, 23:53: PM
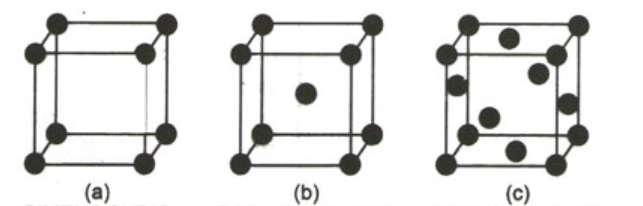
We will be trying to find out the distance between the atoms (edge length, a) and the radius of cation and anion for different crystal structures.
Basically 7 crystal structures are know but we will be referring to the 3 main structures as shown above-
a) Simple Cubic Crystal structure -
in this structure each atom is surrounded by 6 other atoms and thus is the coordination number.
Here distance = Edge Length = a.
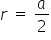
b) Body Centered Cubic Cell -
Fig b represents a BCC Crystal system for this the relation between "r" and "a" is
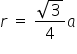
c) Face centered Cubic cell -
Fig c represents a FCC crystal lattice for this the relation is given as
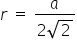
Answered by Sumit Chakrapani | 24 Feb, 2019, 14:56: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













