ICSE Class 10 Answered
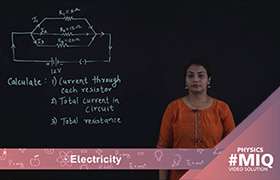
The emf of a cell depends upon:
(i) the material of the electrodes : It depends upon the physical and electrical properties of the electrodes (i.e. their rate of corrosion etc.).
(ii) the electrolyte used is cell: It depends on the concentration of the electrolyte and the temperature at which the chemical reaction takes place.
Taking an example of zinc-copper voltaic cell, When the two plates of the cell are connected by electrical conducting material an electric current flows from copper to zinc in the external circuit. Because of the flow of electrons, the negative potential of the zinc falls and more number of zinc ions move into the solution, again rising its negative potential. When electrons reach the copper its positive potential decreases which is then compensated by more hydrogen ions reaching the copper plate. Electric current will continuously flow until the zinc plate dissolves completely or the sulfuric acid is consumed.