CBSE Class 11-science Answered
What are localised and delocalised types of
Bonding? Explain the difference between them in details.
Asked by Sunil Soni | 07 Sep, 2015, 05:22: PM
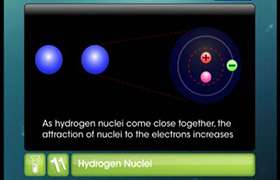
- Chemical bonds are formed when two nuclei share a pair of electrons between them.
- These bonds are considered combinations of overlapping one-electron atomic orbitals.
- The bond is strongest when the two electrons are confined to a region between the two nuclei. This type of bond is described as a localised bond.
- Delocalised bonding electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.
- For example, in Benzene molecule, the delocalisation of electrons is indicated by circle.
- A moleculae with delocalised bonding is more stable than a molecule with localised bonding.
Answered by Prachi Sawant | 08 Sep, 2015, 11:00: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM
CBSE 11-science - Chemistry
Asked by toshan.dandi5 | 24 Feb, 2021, 09:24: PM
CBSE 11-science - Chemistry
Asked by patilanil3766 | 01 Jan, 2021, 10:28: PM
CBSE 11-science - Chemistry
Asked by anjalikri2201 | 08 Aug, 2020, 11:22: AM
CBSE 11-science - Chemistry
Asked by khushipatel2124 | 07 Jul, 2020, 04:23: PM
CBSE 11-science - Chemistry
Asked by saurabhchaddha4205 | 03 Jun, 2020, 04:22: PM
CBSE 11-science - Chemistry
Asked by utkarshnikam85.tl | 06 Apr, 2020, 12:13: PM