CBSE Class 12-science Answered
what are concentration cells?why the oxidation takes place on the electrode with lower conentration in a concentration cell in which both the electrodes are of the same type but the concentrations of ions in them are different?
Asked by Nishtha Sardana | 25 Jul, 2014, 10:11: AM
Dear dollnishtha@yahoo.co.in
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We have not understood the second query that you have posted. We would request you to clarify / provide additional details so that we may answer this to the best of the ability.
However, the answer to your query which we understood is,
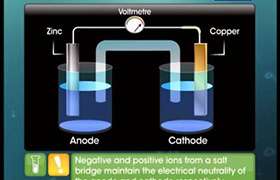
A concentration cell is an electrolytic cell that is comprised of two half-cells with the same electrodes, but differing in concentrations.
The tendency of electrons to flow from one chemical to another is known as electrochemistry. This is what occurs in a concentration cell. The electrons flow from the left side (or left beaker) to the right side (or right beaker). Because the left side is losing electrons and the right is gaining them, the left side is called the oxidation side and the right side is the reduction side. Although you could switch the two to be on the opposite sides, this is the general way in which the set up is done. The oxidation side is called the anode and the reduction side is the cathode. It is the flow of the electrons that cause one side to be oxidized and the other to be reduced.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Hanisha Vyas | 30 Jul, 2014, 02:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM
CBSE 12-science - Chemistry
Asked by Poojanisha1988 | 19 Jul, 2023, 09:59: PM
CBSE 12-science - Chemistry
Asked by jajimuji2306 | 03 Apr, 2022, 01:38: PM
CBSE 12-science - Chemistry
Asked by Harshfarwaha | 23 Jul, 2020, 03:27: PM
CBSE 12-science - Chemistry
Asked by sourabhkumar9923 | 19 May, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by ssharondaniel | 27 Jul, 2019, 06:22: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 12 Sep, 2018, 05:40: PM