CBSE Class 11-science Answered
well i didn't understand the meaning of equilibrium.
Asked by | 05 Oct, 2011, 11:59: PM
Chemical equilibrium is achieved in a reaction when the rate of the forward reaction is equal to the rate of the reverse reaction. When a chemical reaction has reached equilibrium, collisions are still occurring: the reaction is now happening in each direction at the same rate. This means that reactants are being formed at the same rate as products are being formed, and this is indicated by double arrows, 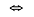 .
.
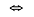 .
.
Answered by | 06 Oct, 2011, 06:01: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by gouravvv641 | 16 Aug, 2022, 09:25: PM
CBSE 11-science - Chemistry
Asked by mangalchandrj79 | 21 May, 2022, 04:38: PM
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by veenatripathi | 28 May, 2020, 09:03: AM










