CBSE Class 12-science Answered
vapour pressure of solution of urea is 736.2 mm at 100 degress celsius calculate osmotic pressure of this solution at 15 degress celsius.
Asked by dhrubajyoti.das | 09 May, 2021, 09:54: PM
Relation between vapour pressure and osmotic pressure-

Answered by Ravi | 13 May, 2021, 04:06: PM
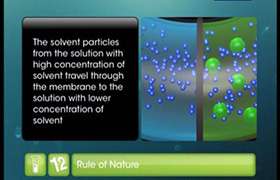
Concept Videos
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by KRISHPATEL.soc | 21 Jun, 2021, 05:58: PM
CBSE 12-science - Chemistry
Asked by dhrubajyoti.das | 09 May, 2021, 09:54: PM
CBSE 12-science - Chemistry
Asked by tiwariaatman | 31 Jul, 2020, 05:10: PM
CBSE 12-science - Chemistry
Asked by yogendrasoni142 | 08 Jun, 2020, 05:43: PM
CBSE 12-science - Chemistry
Asked by santosh357m | 28 Apr, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by Balbir | 27 Jul, 2019, 05:02: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 04 Jun, 2019, 02:28: PM