CBSE Class 10 Answered
Valency of sulphur is 2 negative which I refered from pg no. 51 of class 9 science ncert textbook and oxygen's valency is also 2 negative then how does sulphur dioxide from as only positive and negative ions form a compound then how does 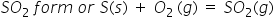
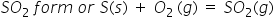
Asked by Varsneya Srinivas | 06 Sep, 2015, 11:06: AM
- Sulphur form higher oxidation state than oxygen as it can utilize all six outer electrons in bonding by promoting some of its to its higher 3d orbital.
- But in case of oxygen, the next empty orbital is 3s which is considerable of higher energy than the highest occupied orbital 2p.
- Oxygen can form positive oxidation state only with fluorine which is highly electronegative.
- Hence, sulphur can show +4 oxidation state in SO2.
- Sulphur show variable oxidation states such as +4,+5, +6 with oxygen in different compounds. Example: SO2, SO3, SO32- , SO42-.
Answered by Prachi Sawant | 06 Sep, 2015, 04:49: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sagarmishra | 04 Mar, 2024, 09:50: AM
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM
CBSE 10 - Chemistry
Asked by prassanna.j | 01 Mar, 2024, 11:59: AM
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 17 Nov, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 07 Jun, 2023, 11:54: AM
CBSE 10 - Chemistry
Asked by shoaibhakak41 | 18 Jan, 2023, 02:17: PM
CBSE 10 - Chemistry
Asked by nehashekh291 | 03 Jan, 2023, 03:11: PM












