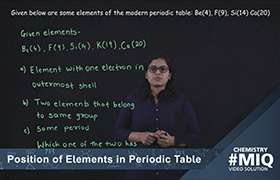
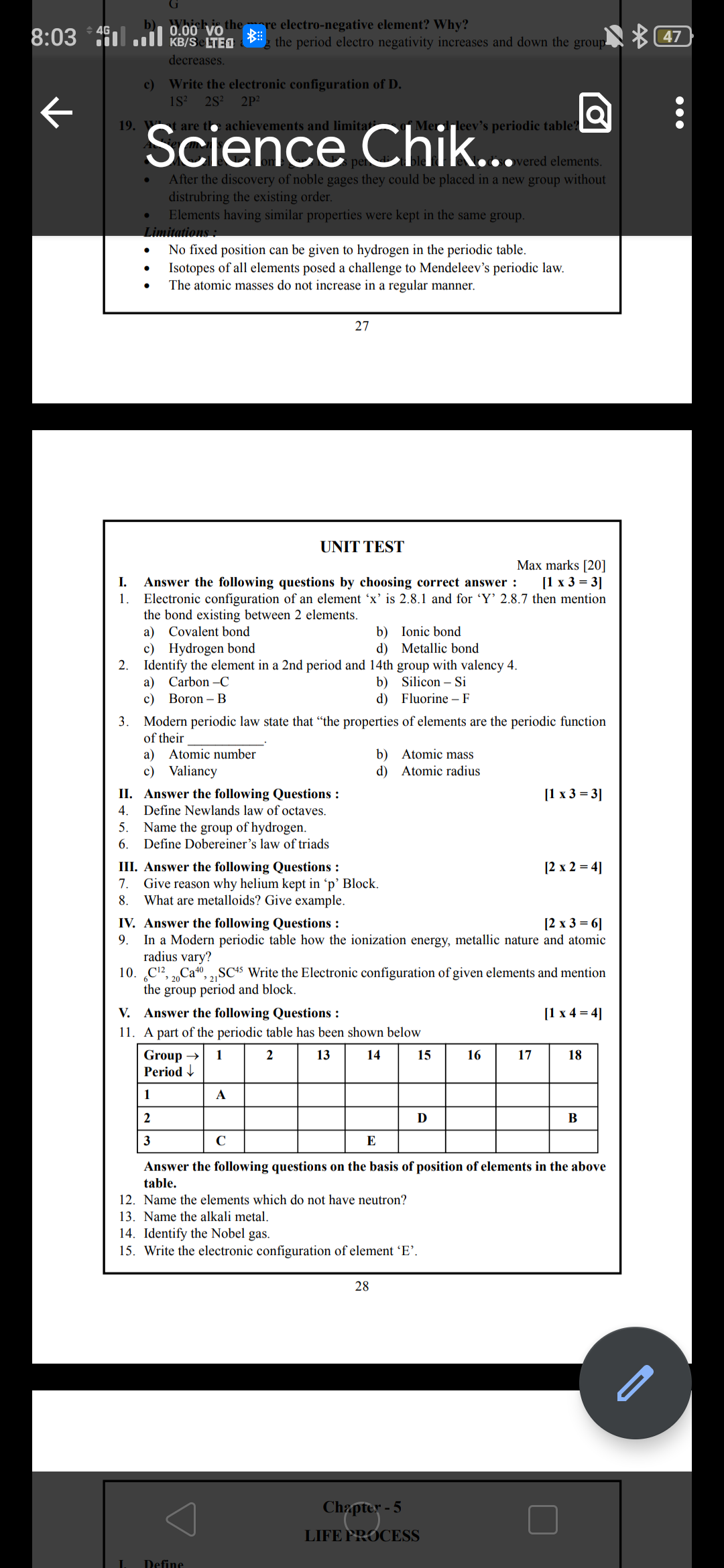
CBSE Class 10 Answered
transition metals
Asked by aashna | 08 May, 2012, 10:59: PM
Yes transition metals possess s, p, d and f orbitals.
Ionisation energy: The energy needed to remove one or more electrons from a neutral atom to form a positively charged ion is a physical property that influences the chemical behavior of the atom. By definition, the first ionization energy of an element is the energy needed to remove the outermost, or highest energy, electron from a neutral atom in the gas phase.
This quantity was formerly called ionization potential, and was at one stage measured in volts. The name "ionization energy" is now strongly preferred.
The electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy when it gains an electron to form a fluoride ion.
| F(g) + e- |
Answered by | 09 May, 2012, 09:25: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM