CBSE Class 11-science Answered
The passage of electric current through silver nitrate and a metal sulphate deposited 4.04 g of Silver and 1.19 g of metal. If the specific heat of metal is 0.095, calculate the atomics mass of metal.
Asked by suryansudash5 | 20 Nov, 2015, 06:41: PM
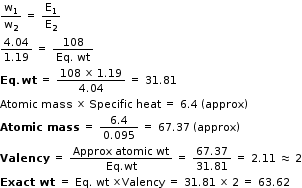
Answered by Arvind Diwale | 23 Nov, 2015, 05:16: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 08:58: PM
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 12:09: AM
CBSE 11-science - Chemistry
Asked by neerudhawanbpsmv | 28 Jun, 2021, 09:22: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 02:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 10:07: PM
CBSE 11-science - Chemistry
Asked by nk581346 | 07 Sep, 2020, 11:08: AM
CBSE 11-science - Chemistry
Asked by swatipuspapatel | 10 Jun, 2020, 06:19: PM
CBSE 11-science - Chemistry
Asked by pratikshyadashrkl | 12 Apr, 2020, 06:47: PM
CBSE 11-science - Chemistry
Asked by devanshuchhipani | 12 Mar, 2020, 08:42: PM









