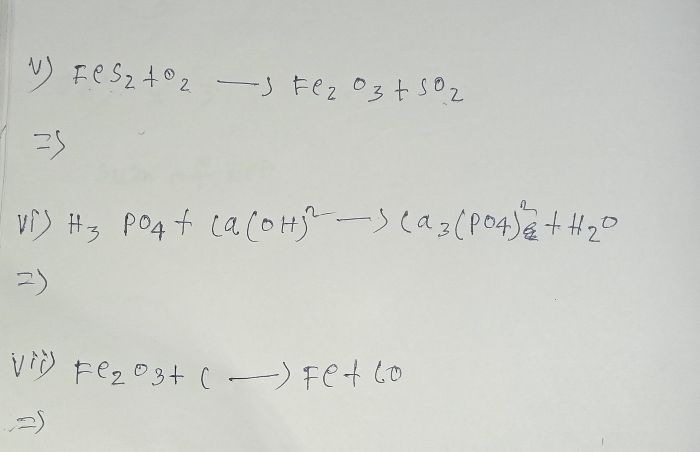CBSE Class 10 Answered
The nitrogen ion have valency 3 then in case of NO3 Why the valency of nitrogen ion is considered to be 5?
Asked by jatin452002 | 07 Jun, 2017, 12:31: PM
In NO3 molecule, the oxidation state of nitrogen is +3 as it gives 3 of its 5 valence electrons form its valence shell for bonding.
Answered by Prachi Sawant | 07 Jun, 2017, 05:21: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM