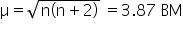CBSE Class 12-science Answered
The magnetic moment (in B.M.) of complex [Co(H2O)6]3+ is??
Asked by sha.bijoy17 | 07 Aug, 2020, 11:55: AM
Co(28) Co2+ = d3
There are 3 unpaired electrons in [Co(H2 O)6]2+ and calculated value of magnetic moment is
Its experimental value is found to be 4.40 BM, due to some contribution of the orbital motion of an electron to the magnetic moment
Answered by Ramandeep | 07 Aug, 2020, 12:39: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by prathyushagn1 | 09 Dec, 2020, 08:12: AM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 31 Aug, 2020, 08:24: PM
CBSE 12-science - Chemistry
Asked by sha.bijoy17 | 07 Aug, 2020, 11:55: AM
CBSE 12-science - Chemistry
Asked by Shambhuhd79 | 22 Jun, 2020, 11:09: AM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Feb, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by smit230503 | 04 Feb, 2020, 08:56: PM
CBSE 12-science - Chemistry
Asked by monishadubey202 | 08 Jan, 2020, 03:42: PM
CBSE 12-science - Chemistry
Asked by Chakshu29saini | 17 Sep, 2019, 06:19: PM
CBSE 12-science - Chemistry
Asked by bjayanta | 24 Mar, 2019, 08:56: PM
CBSE 12-science - Chemistry
Asked by himanshuneb | 28 Jan, 2019, 10:33: PM