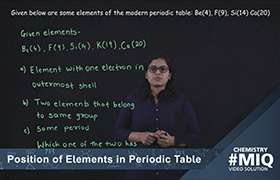
CBSE Class 10 Answered
the m shell has the capacity of holding 18 electrons.then why does potassium have electronic configuration of 2,8,8,1 and not 2,8,9?
Asked by aashiya kanha sharma | 29 Dec, 2013, 01:30: PM
Potassium has 19 electrons and m shell is 3rd energy level.
Its electronic configuration is 1s22s22p63s23p6 4s1 instead of 1s22s22p63s23p6 3d1.
This is because of Afbaus principle which states that electrons fill orbitals starting at the lowest available (possible) energy levels before filling higher levels (e.g. 1s before 2s and 4s before 3d).
As the energy of 3d orbital is higher than 4s, the last electron in potassium get accomadated in 4s energy level instead of 3d.
Answered by Prachi Sawant | 02 Jan, 2014, 12:19: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM