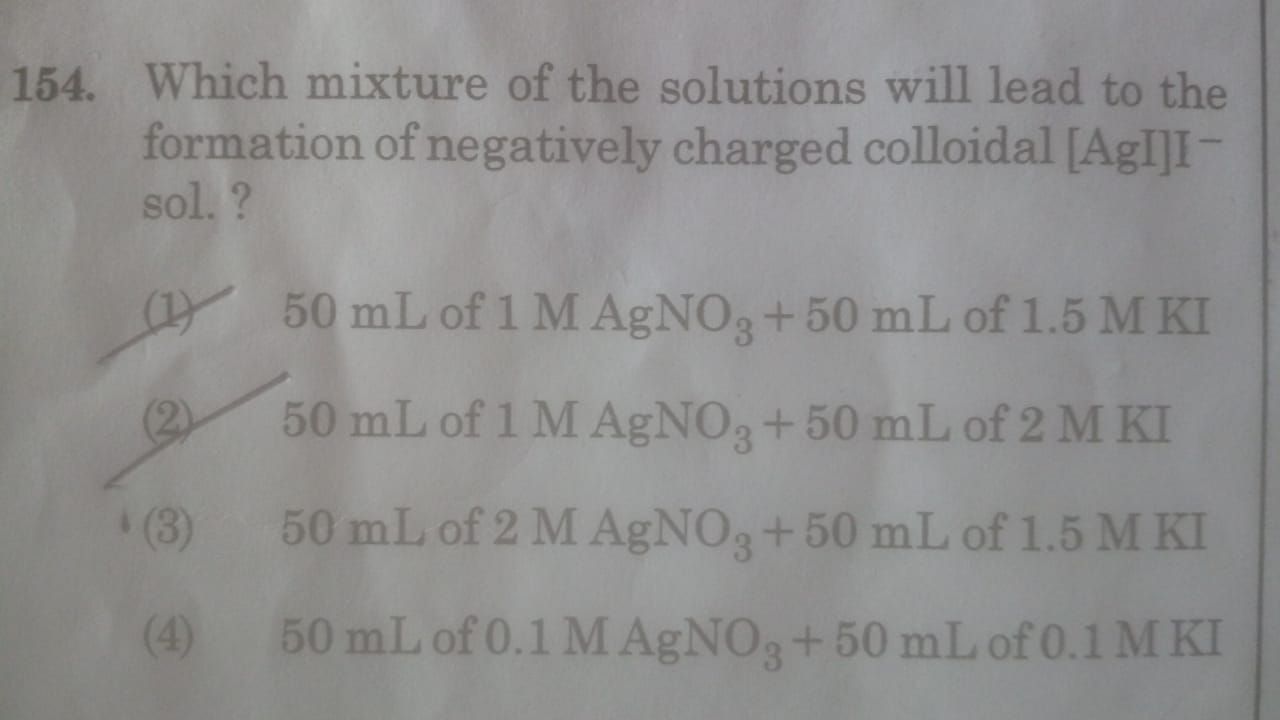CBSE Class 12-science Answered
The colloidal particles___________________the incident ray.
Fill in the blank.
Asked by kpbhake | 23 Oct, 2017, 06:16: PM
The colloidal particles scatters the incident ray.
*Tyndall effect
Answered by Varsha | 24 Oct, 2017, 11:09: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by ntg432000 | 22 May, 2019, 08:10: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 03 Oct, 2018, 08:06: PM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 11:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 21 Jun, 2016, 12:32: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM