ICSE Class 10 Answered
The atomic number of iron is 26
Its electronic configuration =2 8 8 8
Then how its valency is 2+ and3+
Asked by lovemaan5500 | 05 Jan, 2018, 05:09: PM
Iron does not have 8 valence electrons, it only has 2, here's why.
Oxidation state depends upon the valence electrons and valence electrons are the electrons present in the outer most shell of an atom
For Fe, n=4, N shell,
In Iron the electronic configuration of Fe = 1s2 2s2 2p6 3s2 3p6 4s2 3d6
from the electronic configuration and the below diagram you may have a clear idea about valence electrons in the Fe
i.e., Iron has only 2 valence electrons.
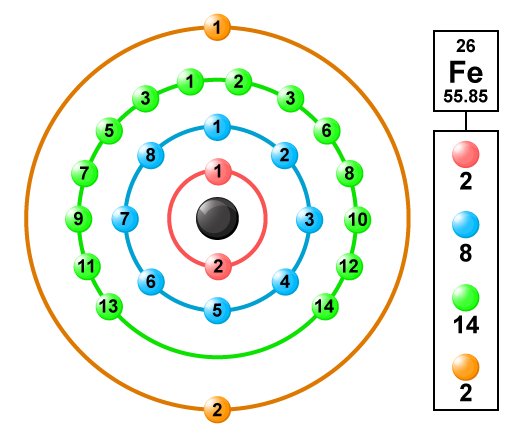
For Fe when two 4s electrons are removed, it has a +2 oxidation state and
electronic configuration of Fe+2 = 1s2 2s2 2p6 3s2 3p6 3d6
Now, the n=3 becomes the outer most shell, iron can lose electrons from this shell as well more specifically from the 3d subshell which has 6 electrons.
when one electron from 3d subshell s removed, iron has a +3 oxidation state and
electronic configuration of Fe+3 = 1s2 2s2 2p6 3s2 3p6 3d5
+2 and +3 are the common oxidation states of Iron.
Answered by Ramandeep | 05 Jan, 2018, 07:22: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by tk5363508 | 01 Apr, 2024, 08:36: PM
ICSE 10 - Chemistry
Asked by sheetal.kolte | 12 Mar, 2024, 10:14: AM
ICSE 10 - Chemistry
Asked by sagarmishra | 12 Mar, 2024, 09:48: AM
ICSE 10 - Chemistry
Asked by anubhutiupadhaya | 11 Mar, 2024, 06:40: PM
ICSE 10 - Chemistry
Asked by sagarmishra | 11 Mar, 2024, 06:36: PM
ICSE 10 - Chemistry
Asked by vijayvijay09644 | 06 Mar, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 05 Mar, 2024, 06:40: PM
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 03 Mar, 2024, 07:07: PM
ICSE 10 - Chemistry
Asked by naaimahhaq | 17 Feb, 2024, 10:54: AM
ICSE 10 - Chemistry
Asked by kundus458 | 07 Feb, 2024, 08:55: AM













