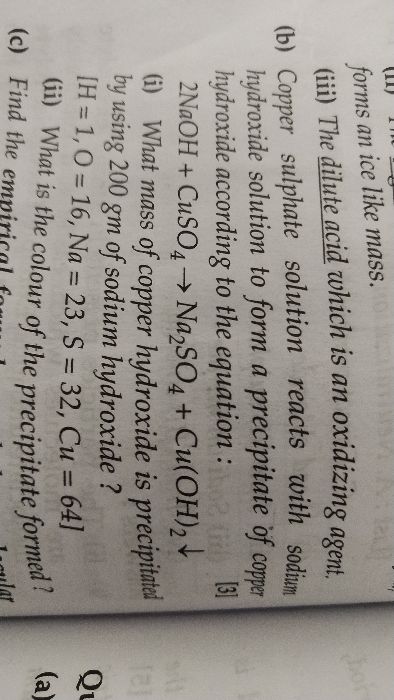ICSE Class 10 Answered
sulphur burns in oxygen to give sulphur dioxide. if 16 g of sulphur dioxide burns in x cc. of oxygen, calculate the amount of potassium nitrate which must be heated to produce x cc of oxygen.
Asked by drashtichauhan75 | 21 Mar, 2019, 07:35: PM
The reaction for burning of sulphur is,

We know,
S = 32, K = 39 , N = 14, O = 16
Moles of S reacted =

0.5 mol of S needs 0.5 mol of Oxygen,
To create confusion, x cc is mentioned in the question, which has no significant use.
So we know that Sulphur burns in 0.5 mol Oxygen.
The thermal decomposition of Potassium nitrate :

2 mols of KNO3 gives 1 mol of O2
0.5 mol of O2 will be produced by 1 mol of KNO3
1mol of KNO3 = 101 g
Therefore the amount of KNO3 required to produce oxygen is 101 g.
Answered by Varsha | 21 Mar, 2019, 09:03: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM